‘Barrier system’ is a term generally used to describe a physical and aerodynamic barrier between the ambient clean room and the area where the fill and closing process of the containers actually happens. In general, we distinguish between two types of barriers:
- Restricted Access Barrier System (RABS)
- Isolators
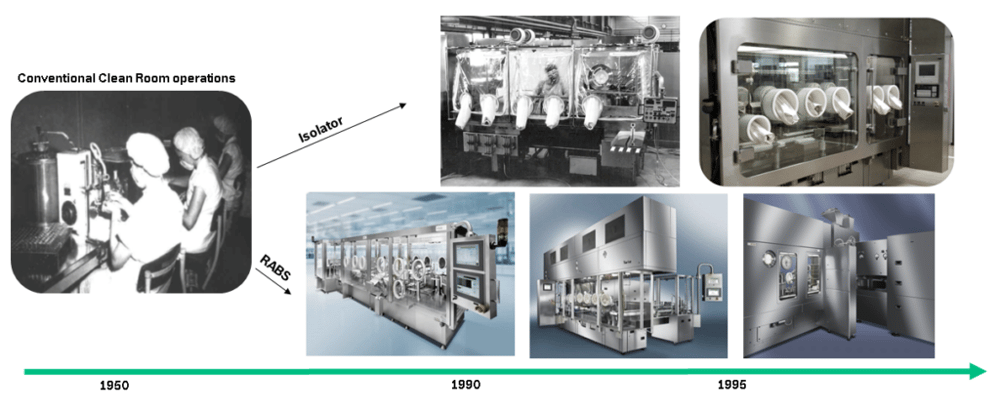
History of barrier systems
Drug manufacturing in the 1950s-70s was far more prone to microbial contamination problems, and the most prominent source for decontamination was identified as human intervention. As a result, the fill-finish process had to be examined more closely and solutions needed to be found to increase the sterility assurance level (SAL). The first installations of passive open RABS began in the late 70s.
Another evolutionary step was made towards active open RABS designs to gain better control of the airflow and to eliminate room design restrictions. The early open RABS installations were not independent from ambient room conditions. Controlling the temperature at point of fill wasn’t possible without influencing the room. In the late 1980s the first closed RABS were installed, which came with their own air-handling units. This made temperature, laminar flow, humidity and pressure control independent from the ambient room. The late 80s, early 90s also brought up the Isolator technology – a closed RABS design – but additionally to standard cleaning procedures with an integrated and automated sanitization process (e.g. VHP – Vaporized Hydrogen Peroxide). Another advantage of closed systems, like cRABS, isolators or even containment isolators, is that the confidence level of safety in dealing with toxic substances is rising.
What are the factors we need to take a closer look at?
The drug specification (e.g. value, OEL, temperature-, oxygen- sensitivity) and risk assessments play a crucial role in deciding on either isolator or RABS. Facility design, air management and pressure concepts, and clean room specifications are impacted by these factors. In older facilities, restrictions of space requirements are most likely affecting decisions. Batch size and frequency of production must also be considered. The increase of sterility assurance and quality in drug fill-finish process is hard to put in figures, but the overall level confidence is not negligible.
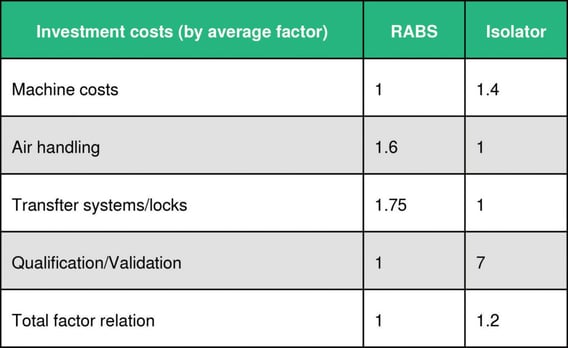
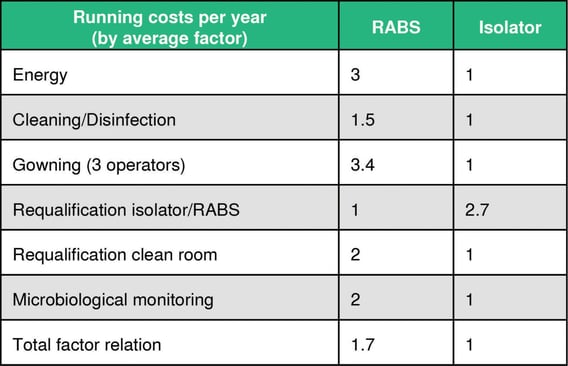
Investment costs and running costs per year
The break-even point in operating an isolator system is reached after between 1.5-4 years, depending on frequency of batches and campaigns.
Facts & Figures
- 50% of the US installations are already Isolators, 40% are RABS and 10% are traditional clean room applications
- Traditional clean room applications are becoming history
- Isolators continue to gain a greater percentage of overall barrier applications world-wide
- RABS systems are more and more treated like Isolators (transfer, glove interventions, etc.) without having the full span of advantages of an Isolator
- Regulators lean more and more towards Isolators
- The higher initial investment for an isolator can be covered well by lower running costs compared to RABS
- The future brings more fully isolated processes, reduction / elimination of human intervention, gloveless systems. Automation and more autonomous processes combined with monitoring and robot technology.
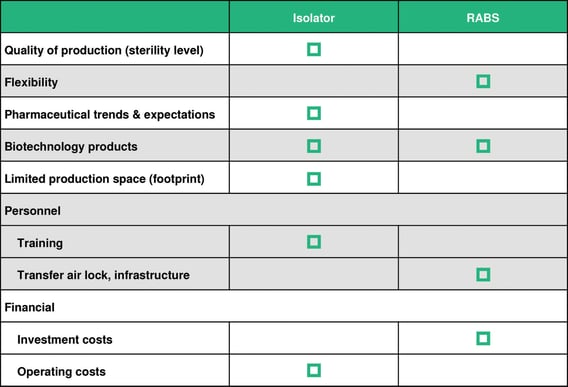
The table below shows the advantages of either system: