In many of my previous Syntegon articles, I have lamented the high cost of drug product development, with good reason. Developing an oral-solid-dose drug product is an expensive and risky endeavor, with formulation and process development being among the most significant and time consuming challenges.
It is no secret that formulation scientists face a host of hurdles when developing a formulation bound for tablet compression. First and foremost is achieving the desired release profile of the tablet, whether immediate release or modified. In addition, API characteristics must be considered when choosing excipients for the blend, including particle size distribution of the API itself, as well as the excipients with which it will be blended. Further, depending on the solubility of the API, solubilizing agents might demand consideration. These are just a few of the many challenges facing the formulation team.
Not to be forgotten when developing a tablet formulation is how the blend will perform on the tablet press. Of course, proper flow of the blend on the press is critical to achieving consistent tablet weights, in addition to the formulation being robust enough to meet hardness and disintegration targets. However, the press itself is of significant importance as well. Ideally, your tablet press will be capable of using multiple tooling options during early development, while also being able to seamlessly scale up to clinical supplies and small-scale production batches.
All of this can be rather daunting even to a well-established company. To a small to medium company with limited facilities and resources, tablet development can feel like a herculean challenge. Moreover, as most experienced development personnel know, delays in formulation or process development mean extra resources and more money.
BIOGRUND
BIOGRUND can help ease the pain of developing both your tablet formulation and your tableting process. BIOGRUND specializes in helping pharmaceutical, nutraceutical and cosmetic companies develop robust OSD formulations by using their specialized line of excipients. Founded in Germany in 1999, BIOGRUND expanded to the US in 2014, relocated to Kentucky in 2019, and now has a facility that will not only help you develop a new formulation, but can also improve your current formulation and manufacturing process. In addition to offering formulation development using their large portfolio of individual excipients, BIOGRUND also offers ingredient premixes such as BonuTab®, a fully formulated premixed tablet and capsule formulation. Using one of BIOGRUND’s ingredient premixes not only saves time in developing your formulation, it also means you need less space to store a large number of excipients, your operations personnel spend less time dispensing material and your analytical personnel spend less time testing multiple excipients. Instead, you store, dispense and test one material.
Syntegon
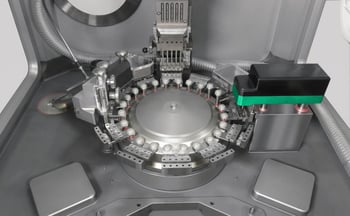
For tableting, BIOGRUND US, a Syntegon partner lab, uses exclusively the TPR 200 tablet press from Syntegon. The TPR 200 can be used to tablet small development batches, using an R&D B/D turret, or can utilize a turret of 32 stations with output of nearly 230,000 tablets per hour. Because all TPR 200 turrets utilize the same pitch circle diameter, scale up is quick and easy.
For more information on how BIOGRUND can help with your formulation, visit their product page here. For more information on Syntegon’s TPR 200 tablet press, visit our product page.
Tom Myers, Business Development Manager, manages our capsule filling lines. He graduated from Loyola University, Chicago and has worked in pharmaceutical manufacturing/operations for over 30 years for companies such as Abbott Laboratories, Genentech, Boehringer-Ingelheim and Biogen.