Materia Medica Holding is one of the top 5 manufacturing pharmaceutical companies for OTC-products in the Russian Federation. For more than 20 years, the company is active in R&D, production and marketing of their own innovative OTC pharmaceuticals for therapeutic fields like antivirus, neurological, gastroenterological, men’s health and more. Currently there are over 20 well established brands in their company portfolio with activities in 16 countries worldwide.
When Materia Medica Holding was faced with the task to implement a Track&Trace infrastructure for their manufacturing plant, the company turned to their trusted partner: Syntegon.
Located in the city of Chelyabinsk, the plant of Materia Medica Holding is GMP-certified by several European agencies and equipped with high-tech equipment from leading manufacturers. It was clear from the start that the Track&Trace infrastructure for the production facility also had to be measured against these high standards.
The regulatory standards for Track&Trace in the Russian Federation have been described as one of the toughest in the world. In addition to a highly complex data matrix code and strict requirements for data integrity, the legal and technical necessities were constantly adapted up to the final deadline of July 1, 2020. This posed a challenge for both the pharmaceutical companies and for the providers of Track&Trace solutions.
The challenge
The joint Materia Medica and Syntegon project team faced a difficult challenge: Four different high speed packaging lines needed to be integrated. Since Materia Medica Holding is a dynamically developing company, the possibility to easily connect additional lines in the future had to be considered. Unified hardware and user interfaces for the different high-speed lines for the production of solid and liquid pharmaceuticals were key requirements. The implemented solution had to be measured against strict productivity requirements with the goal to keep the loss of productivity caused by the Track&Trace processes as close to zero as possible. Since quality of their products is of the utmost importance for Materia Medica, checkweighers and applicators for tamper evident labels had to be integrated into the serialization equipment. The aggregation operations in shipper cases and on pallets needed to be performed manually directly at the production lines, with the future ability to easily implement completely automated solutions. For post-production re-work, a separate workstation in the warehouse was necessary.
The complete Track&Trace solution had to be interfaced and compatible with the customer‘s ERP-system and deliver the data quality and formats, required by both Materia Medica and by the Russian government legislation. And last but not least, the complete system of hardware and software solutions had to be equipped with interfaces in Russian language.
The solution
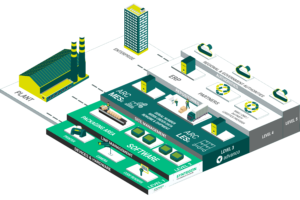
The Syntegon Open Serialization Platform is covering all the above mentioned customer demands - and even more: This integrated innovative and high quality solution from Level 1-3 is the result of Syntegon’s longstanding experience in the field of packaging machinery and the flexible CPI software solution with over 30 implemented installations all over the world.

Solution components
Hardware modules:
CPS 1900 WTE – serialization module with integrated checkweigher and tamper evident label applicators for line speeds of up to 300 cartons per minute is the best solution to have three functionalities in one machine with a minimal footprint. The highest machine quality ensures a longevity, minimal maintenance and training efforts and low reject rates.
CPA 0410 CP – manual aggregation station for aggregation of cases and pallets with auto-focus system for layer reading. This station provides an easy user experience because since the operator is guided by status lights and provided displayed information. There is no need to adjust the distance between the camera and the working table at anytime — no matter how many layers are packed in a case or for different case sizes, it’s always the same distance.
CPA 0110 re-work station – the workstation with an installation of the CPI rework client software is required for production related re-work operations if the batch is already closed at the line and all data is uploaded to upper systems. Besides state change (good, bad, sample) operators are able to aggregate, de-aggregate and re-aggregate. It is very useful, for example, if a pallet is broken due to any reason.
CPI software – Syntegon’s own L3 software solution with extensive Track&Trace functionality offers not only serial number management, but material management as well. It includes many additional functionalities beside Track&Trace. For example, it can also be equipped with the management cockpit, which provides a quick live overview of all lines, and can be expanded in the future quite easily.
The result
The system was implemented months before the official set deadline for the serialization project in Russia (July 1, 2020). From the moment of installation, both the system and the connection to the governmental MDLP system were continuously and intensely tested. From July 1, all production has been marked and all data has been transmitted to the government system MDLP. Since then, the Syntegon Track&Trace system has been running smoothly. As promised, it fulfilled all requirements set by Materia Medica Holding. The Syntegon system is giving Materia Medica Holding a competitive edge with an open system, readily adaptable for further tasks and requirements. As the company is developing, the Syntegon Track&Trace platform is growing with it. In case of necessary support, the global Syntegon service network has specialists locally available that are helping Materia Medica Holding to keep downtimes to a minimum.