
Service Agreements
Find out more about our Service Agreements here.
Syntegon offers internal and external cleaning machines for vials and other containers. Once the vials have been pre-cleaned with Water for Injection (WFI), our HQL tunnel ensures safe and reliable sterilization and depyrogenation with dry heat.
Learn more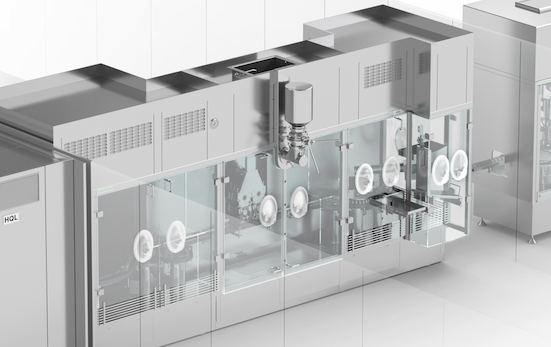
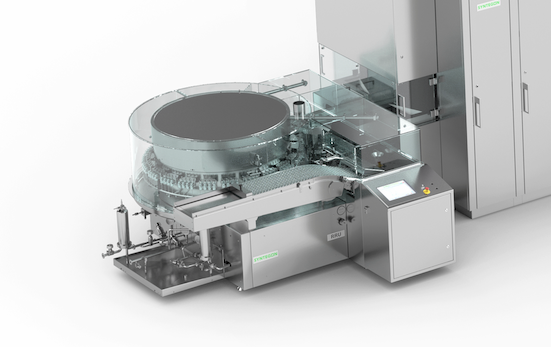
A wide range of technologies, including valve piston and peristaltic pump, mass-flow and time-pressure filling, make sure you can fill your products in a highly customized way. Tool-less format changes and filling stations with a variable number of filling heads increase the flexibility of your filling processes.
Learn more
Capping Continuous capping with high speeds completes the safe and reliable closing process. 100 % in-process control (IPC) check weighing and inline inspection systems for empty vials, stopper position or crimp quality further increase product quality.
Learn more
Following filling and capping, the vials can be transferred to our RAN external cleaning machine, which makes sure that no particles adhere to the outer sides of the vials.
Learn more
Barrier Systems Syntegon has established a comprehensive portfolio, including open RABS, closed RABS, containment and isolator systems.
Learn more
State-of-the-art technologies for visual inspection and container closure integrity testing (CCIT), including high voltage leak detection (HVLD), static division (SD) and headspace analysis (HSA).
Learn morePharmaceutical powders form the basis of numerous antibiotics and other medicines that are essential for comprehensive medical care. The finely ground-active ingredients generate dust easily. Hence, machine operators must be protected from these sometimes toxic or potent ingredients. Syntegon has been supporting customers in pharmaceutical powder filling for more than 60 years. Our experts will be happy to pass on their expertise to you, too!

Pure media are essential utilities in parenteral pharmaceutical manufacturing. From “cold” membrane-based systems for the generation of PW, HPW, and WFI and “hot” distillation-based systems for the production of WFI and PS, to the corresponding storage systems and distribution to the point of use, our portfolio provides a comprehensive and seamlessly integrated pure media infrastructure. For your drug product compounding needs, in turn, we also offer formulation systems. Comprising a wide range of process vessels and their automation as well as all associated peripherals including media supply and CIP/SIP units, our modular processing systems truly are “the perfect match for every batch.”

Sterility matters across the entire aseptic process – before, during and after sterile filling. Syntegon offers internal and external cleaning machines for vials and other containers. Once the vials have been pre-cleaned with Water for Injection (WFI), our HQL tunnel ensures safe and reliable sterilization and depyrogenation with dry heat. Following filling and capping, the vials can be transferred to our RAN external cleaning machine, which makes sure that no particles adhere to the outer sides of the vials; an important factor in protecting operators and healthcare workers.

Our vial filling systems combine flexibility and precision: a wide range of technologies, including auger dosing, pressure vacuum, or slide chamber dosing, make sure you can fill your products in a highly customized way. Tool-less format changes and filling stations with a variable number of filling heads increase the flexibility of your filling processes. Subsequent sealing offers you a variety of methods such as pick and place or continuous stoppering. Continuous capping with high speeds completes the safe and reliable closing process. 100 % in-process control (IPC) check weighing and inline inspection systems for empty vials, stopper position or crimp quality further increase product quality.

Aseptic filling is all about product safety – and thus the separation of operators and pharmaceutical ingredients. Isolator technology helps to build this crucial barrier. Syntegon has established a comprehensive portfolio, including open RABS, closed RABS, containment and isolator systems. All solutions are tailored to customer requirements, from machine design and development to on-site installation and validation.

Like any pharmaceutical container, vials require thorough inspection to ensure patient safety. Our wide range of manual, semi and fully automated inspection machines inspects liquid and lyophilized pharmaceuticals for particles and container defects. Thanks to our long-standing inspection expertise, we offer state-of-the-art technologies for visual inspection and container closure integrity testing (CCIT), including high voltage leak detection (HVLD), static division (SD) and headspace analysis (HSA). Moreover, Syntegon provides fully integrated inline inspection solutions for several inspection tasks such as empty vial, crimping and stopper seat inspection.


Find out more about our Service Agreements here.

Find out more about Digital Solutions here.

Find out more about Parts here.

Find out more about Maintenance here.
/remote-assistant.jpg?width=640&height=380&name=remote-assistant.jpg)
Find out more about Technical Support here.

Find out more about Modernizations here.

Find out more about our Training here.

Find out more about our Expert Services here.
Discover Syntegon, a leader in vaccine manufacturing equipment with over 30 years of experience and 200 successful projects worldwide.
Explore Syntegon's transition from Waiblingen to new locations, setting the stage for future growth...
Join us this June for our OSD Academy expert seminar “Granulation – from powder to tablet”.
Learn more about our sustainable coffee packaging solutions.
Join us at Pharmatag 2025 in Crailsheim, Germany, and experience how co-creation revolutionizes...