
Service Agreements
Find out more about our Service Agreements here.
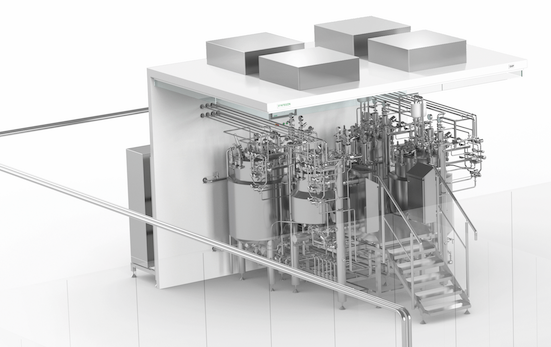
When it comes to bioprocessing, the systems from Syntegon’s subsidiary Pharmatec flexibly support the production of small and large volume parenterals (SVP/LVP) and infusions (IV).
Learn more
With highly automated bag and tub opening solutions for ready-to-use containers, we help you process pre-filled syringes both safely and efficiently.
Learn more
Processing pre-filled syringes with expensive parenteral drugs requires utmost care and precision. Syntegon’s comprehensive filling and closing systems meet these requirements.
Learn more
As experts in nested syringe operations, we have developed exclusive solutions for the highly automated de-nesting and secure positioning of pre-filled syringes before downstream processing.
Learn more
With the Rod Inserter and Labeler (RIL), plunger rod insertion and label application are realized at high speeds for both glass and plastic (COC) syringes, ensuring sterile downstream handling at its best.
Learn more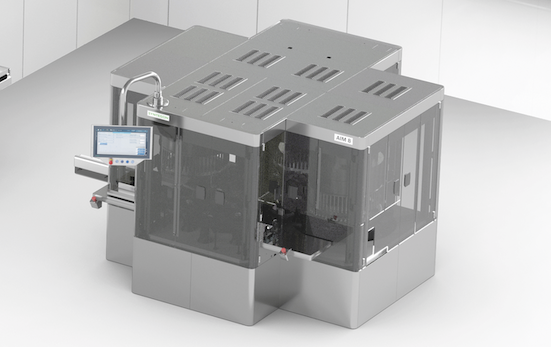
We offer state-of-the-art technologies for visual inspection and container closure integrity testing (CCIT), including high voltage leak detection (HVLD), static division (SD) and headspace analysis (HSA).
Learn more
The Syntegon company SBM (Schoeller-Bleckmann Medizintechnik) offers a vast portfolio of versatile sterilization solutions for effective treatment of porous loads and liquids in open, semi-sealed or closed containers.
Learn moreSyringes are the go-to packaging for many high-potent parenteral drugs, including biopharmaceuticals. We offer you a large variety of fully automated filling lines that ensure maximum product safety and process flexibility – from single pack styles and filling technologies to a full combi line, from small batches to high outputs.

When it comes to bioprocessing, the systems from Syntegon’s subsidiary Pharmatec flexibly support the production of small and large volume parenterals (SVP/LVP) and infusions (IV). The process solutions comprise modular set-ups of temperature-controlled stainless-steel tanks that can be assembled to meet customer-specific settings.

Whether you run a small, medium, or high output line, safe processes are paramount to maintaining product quality. With highly automated bag and tub opening solutions for ready-to-use containers, we help you process pre-filled syringes both safely and efficiently. Especially in nested syringe application settings, our ABO automatic bag openers and ATO automatic tub openers minimize operator contact and contamination risks through fully automatic, aseptic removal processes.

Processing pre-filled syringes with expensive parenteral drugs requires utmost care and precision. Syntegon’s comprehensive filling and closing systems meet these requirements, and many more: flexible handling allows for a gentle removal from the nest, while in-process control (IPC) further improves filling quality through essential filling weight and stopper location measurements. The wide variety of filling systems, including peristaltic pumps, add to the overall process hygiene. Vent tube or vacuum stopper insertion makes sure the syringes are tightly sealed.

Our pre-filled syringe lines make sure syringes are handled with care right from the start. Dedicated handling units allow syringes to be precisely removed from nests and tubs without operator intervention. As a result, individual syringes can be fed into integrated inspection stations easily and without contamination. As experts in nested syringe operations, we have developed exclusive solutions for the highly automated de-nesting and secure positioning of pre-filled syringes before downstream processing. The devices can be effectively combined with re-nesting and inspection platforms.

The syringe process does not stop after filling. Instead, it needs further sophisticated steps from rod insertion to applying visible, easily identifiable labels to the outside. We combine these two steps in a single platform: with the Rod Inserter and Labeler (RIL), plunger rod insertion and label application are realized at high speeds for both glass and plastic (COC) syringes, ensuring sterile downstream handling at its best.

Like any pharmaceutical container, syringes require thorough inspection to ensure patient safety. Our wide range of manual, semi and fully automated inspection machines inspects liquid and lyophilized pharmaceuticals for particles and container defects. Thanks to our long-standing inspection expertise, we offer state-of-the-art technologies for visual inspection and container closure integrity testing (CCIT), including high voltage leak detection (HVLD), static division (SD) and headspace analysis (HSA). Moreover, Syntegon provides fully integrated inline inspection solutions for several inspection tasks such as defects detection and stopper seat inspection.

The Syntegon company SBM (Schoeller-Bleckmann Medizintechnik) offers a vast portfolio of versatile sterilization solutions for an effective treatment of porous loads and liquids in open, semi-sealed or closed containers. The vacuum-steam, steam/air mixture and hot water shower processes support you in achieving reproducible equipment and terminal sterilization while optimizing your energy requirements.


Find out more about our Service Agreements here.

Find out more about Digital Solutions here.

Find out more about Parts here.

Find out more about Maintenance here.
/remote-assistant.jpg?width=640&height=380&name=remote-assistant.jpg)
Find out more about Technical Support here.

Find out more about Modernizations here.

Find out more about our Training here.

Find out more about our Expert Services here.
Discover Syntegon, a leader in vaccine manufacturing equipment with over 30 years of experience and 200 successful projects worldwide.
Explore Syntegon's transition from Waiblingen to new locations, setting the stage for future growth...
Join us this June for our OSD Academy expert seminar “Granulation – from powder to tablet”.
Learn more about our sustainable coffee packaging solutions.
Join us at Pharmatag 2025 in Crailsheim, Germany, and experience how co-creation revolutionizes...